Application Notes


Transitioning to fully defined HiDef-B8 for adherent human pluripotent stem cell cultures
This application note demonstrates that human pluripotent stem cells (hPSCs) can be efficiently transitioned from high-protein, albumin-rich media such as mTeSR™ Plus to the fully defined, animal-free HiDef® B8 medium using either direct or gradual adaptation workflows. The study provides practical, validated procedures for both adherent ESCs and iPSCs, including a five-day stepwise approach that maintains healthy morphology, stable growth, and robust pluripotency throughout the transition. Cells adapted to HiDef-B8 exhibit the expected shift to a looser, “continent-like” colony morphology while retaining high viability and expression of pluripotency markers, with genomic stability supported over extended passaging as previously published.
The guide further highlights how Ready-CEPT™ enhances survival and consistency during enzymatic or single-cell workflows, improving outcomes during adaptation and enabling gene-editing and clonal expansion applications. Key considerations, including recommended hypoxic culture conditions, ideal confluence, passaging strategies, and substrate compatibility, ensure reproducible performance across diverse hPSC lines. Developed to overcome the variability, cost, and animal-derived components found in legacy media systems, HiDef-B8 offers a chemically defined, serum-free platform optimized for long-term, weekend-free adherent hPSC maintenance. By simplifying workflows, reducing undefined proteins, and enabling seamless movement toward translational and cGMP-aligned processes, HiDef-B8 provides researchers with a reliable, scalable foundation for modern human pluripotent stem cell culture.
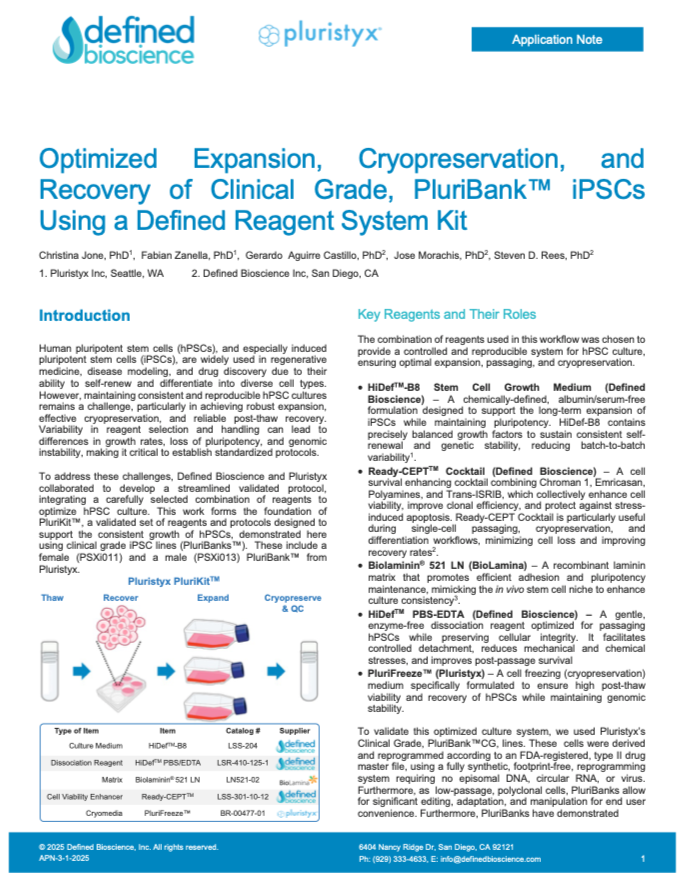
Optimized Expansion, Cryopreservation, and Recovery of Clinical Grade, PluriBank iPSCs Using a Defined Reagent System Kit

Comparative Analysis of 2D and 3D Differentiation Approaches for iPSC-Derived Neural Cells
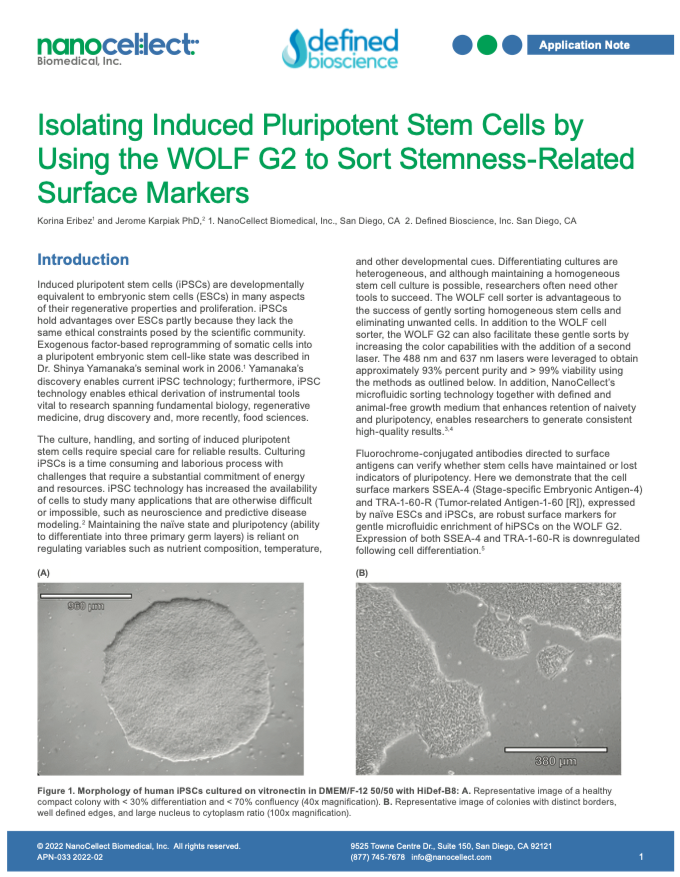
Isolating Induced Pluripotent Stem Cells by Using the WOLF G2 to Sort Stemness-Related Surface Markers
Posters

High-density Pluripotent Stem Cell (iPSC) Expansion in a Single-use Bioreactor using HiDef® S8
This poster highlights a collaborative bioreactor workflow for high-density suspension expansion of human pluripotent stem cells using HiDef® S8. We demonstrate consistent aggregate formation, rapid five-day expansion to more than seven billion cells, and seamless return to 2D culture with maintained pluripotency and differentiation potential. This platform provides a scalable, animal-free solution for efficient large-volume hPSC manufacturing. Presented at BMES 2025.
iPSC Expansion in a Stirred-Tank Bioreactor: Optimization and Automation of Feeding Strategies to Achieve Economic Feasibility
This poster presents a comparative bioreactor study led by IST Lisbon using HiDef® S8 to evaluate media performance, automated feeding strategies, and cost efficiency in stirred-tank iPSC expansion. S8 supported faster growth, higher TRA-1-60⁺ yields, and superior pluripotency maintenance at substantially lower media usage and cost. The work highlights S8 as a high-density, economically favorable suspension medium suitable for scalable 3D iPSC manufacturing and downstream applications. Presented at BMES 2025.

Growth factor production for enhanced growth of fish multipotent stem cells for cell-cultivated meat
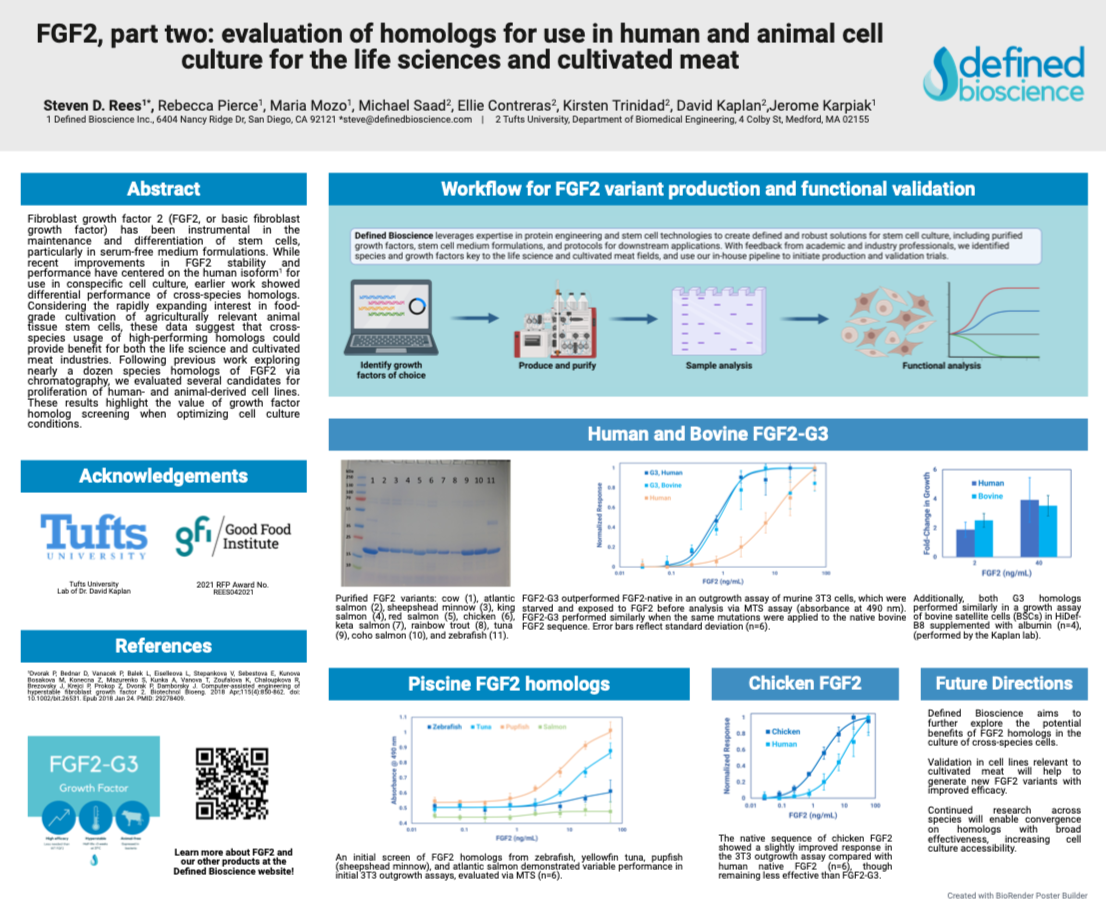
FGF2, part two: evaluation of homologs for use in human and animal cell culture for the life sciences and cultivated meat

Defined cGMP animal-origin-free medium supports PSC during, and promotes single-cell clonal expansion after, microfluidic sorting
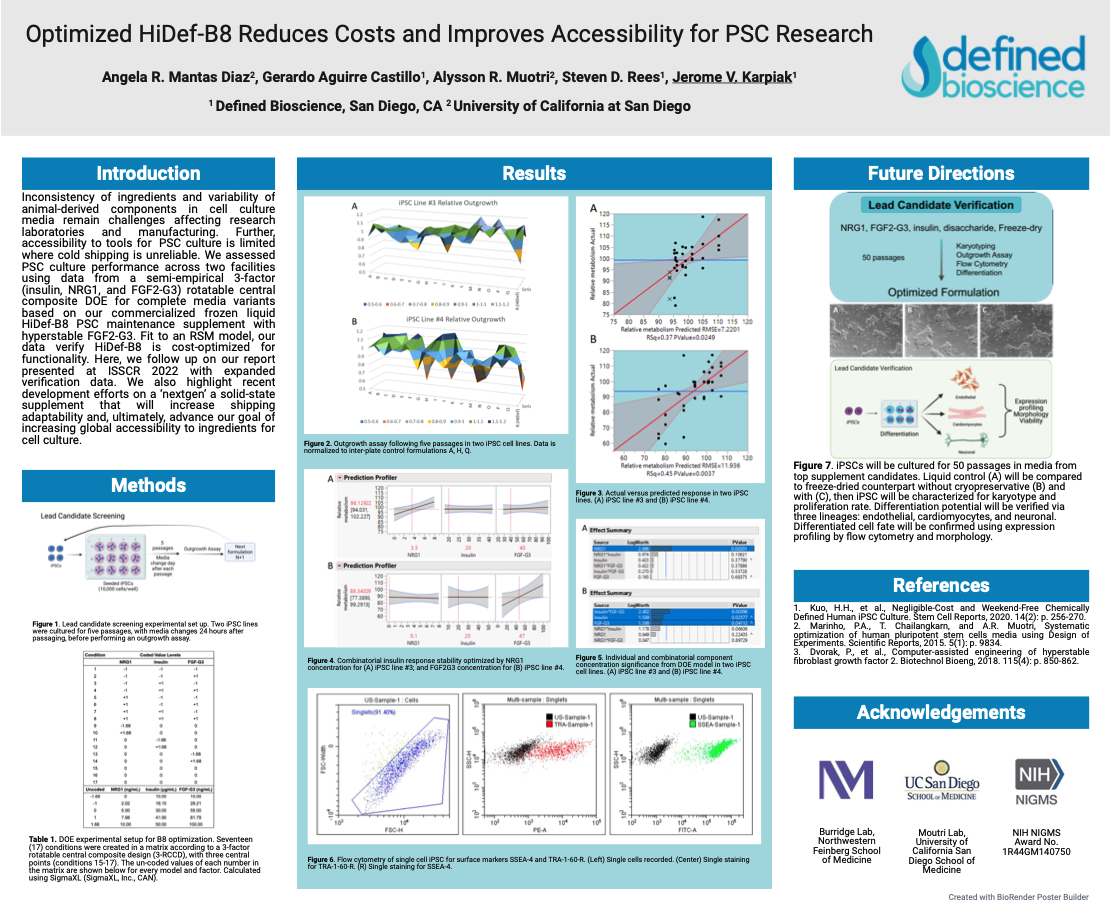
Optimized HiDef® B8 Reduces Costs and Improves Accessibility for PSC Research

Development of FGF2 homologs for applications in human stem cell culture and the cultivated meat industry
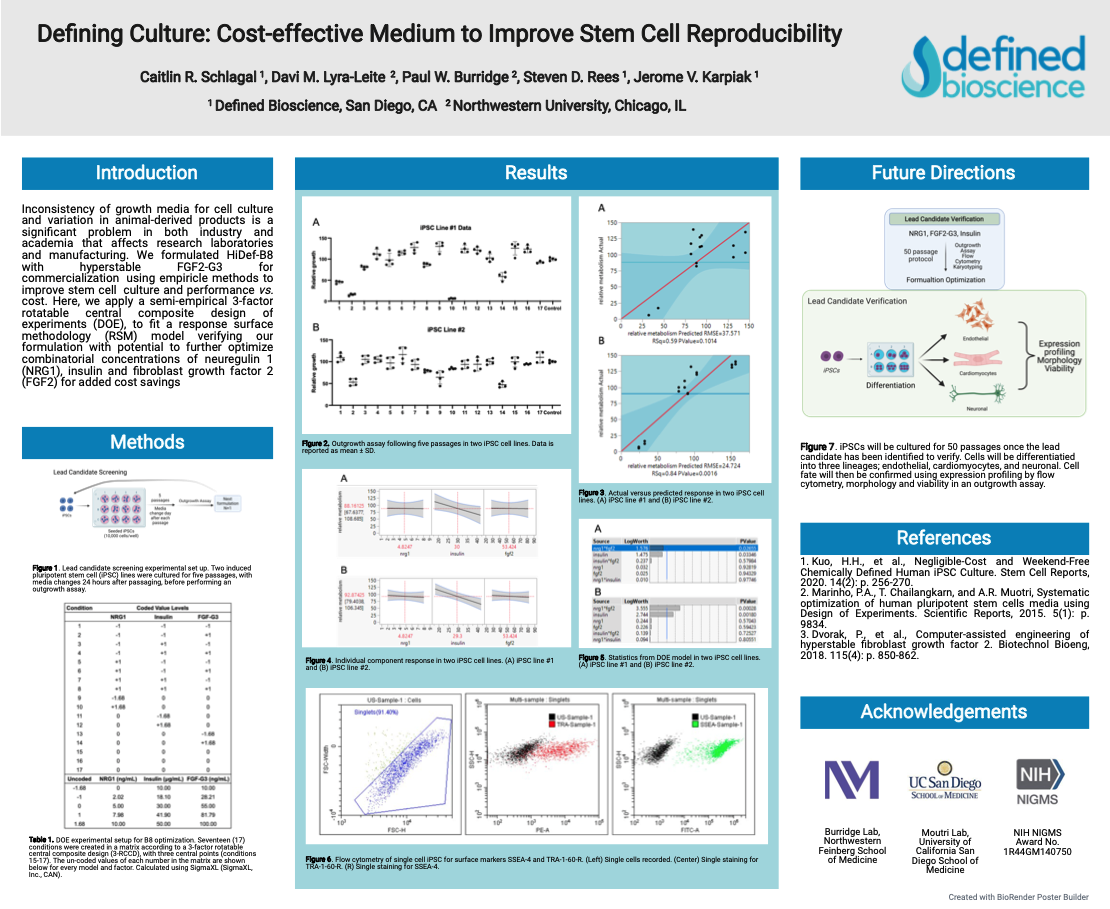
Defining Culture: Cost-effective Medium to Improve Stem Cell Reproducibility

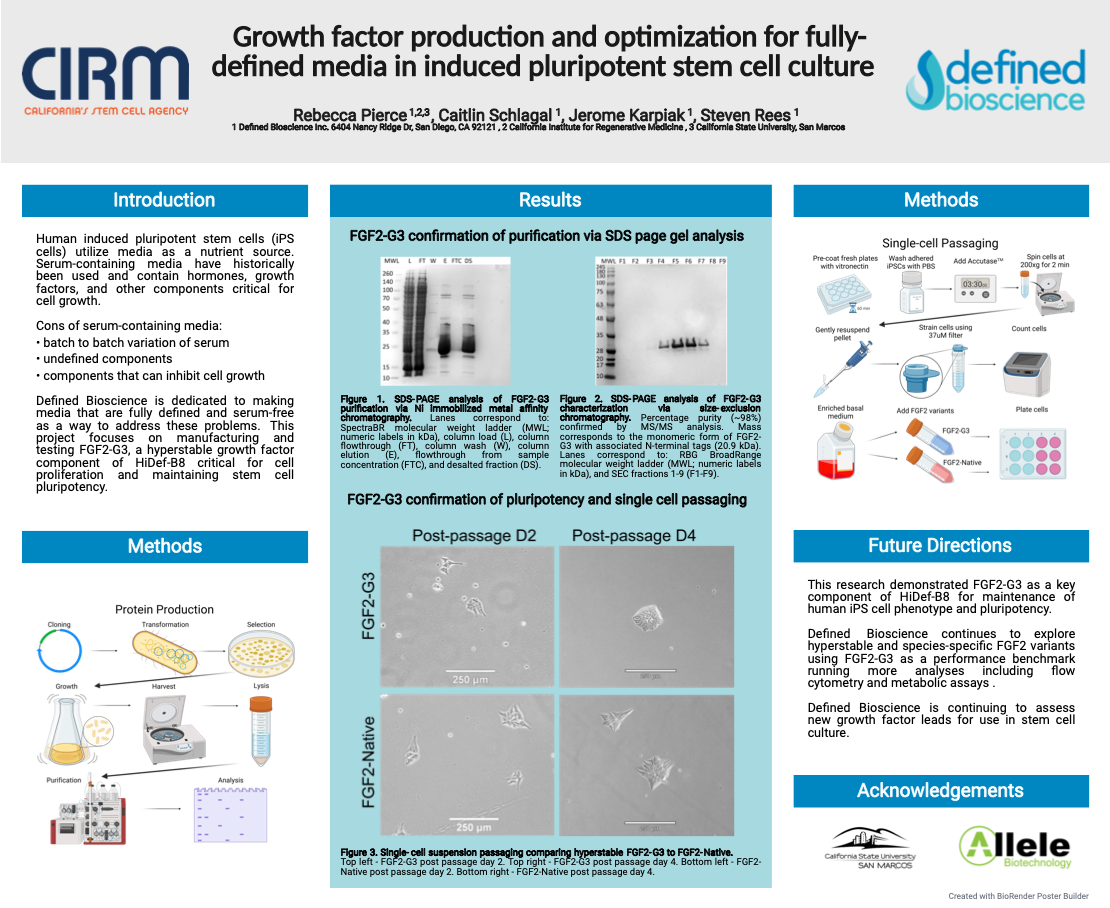
Growth factor production and optimization for fully-defined media in induced pluripotent stem cell culture
Let’s Collaborate on What’s Next
We welcome partnerships with biomedical innovators, biotech leaders, and research institutions. If you're shaping the next breakthrough, let’s collaborate.