Optimized Expansion, Cryopreservation, and Recovery of Clinical Grade, PluriBank iPSCs Using a Defined Reagent System Kit

Pluristyx Inc, Seattle, WA
- Christina Jone, PhD
- Fabian Zanella, PhD

Defined Bioscience Inc, San Diego, CA
- Gerardo Aguirre Castillo, PhD
- Jose Morachis, PhD
- Steven D. Rees, PhD
Human pluripotent stem cells (hPSCs), and especially induced pluripotent stem cells (iPSCs), are widely used in regenerative medicine, disease modeling, and drug discovery due to their ability to self-renew and differentiate into diverse cell types. However, maintaining consistent and reproducible hPSC cultures remains a challenge, particularly in achieving robust expansion, effective cryopreservation, and reliable post-thaw recovery. Variability in reagent selection and handling can lead to differences in growth rates, loss of pluripotency, and genomic instability, making it critical to establish standardized protocols.
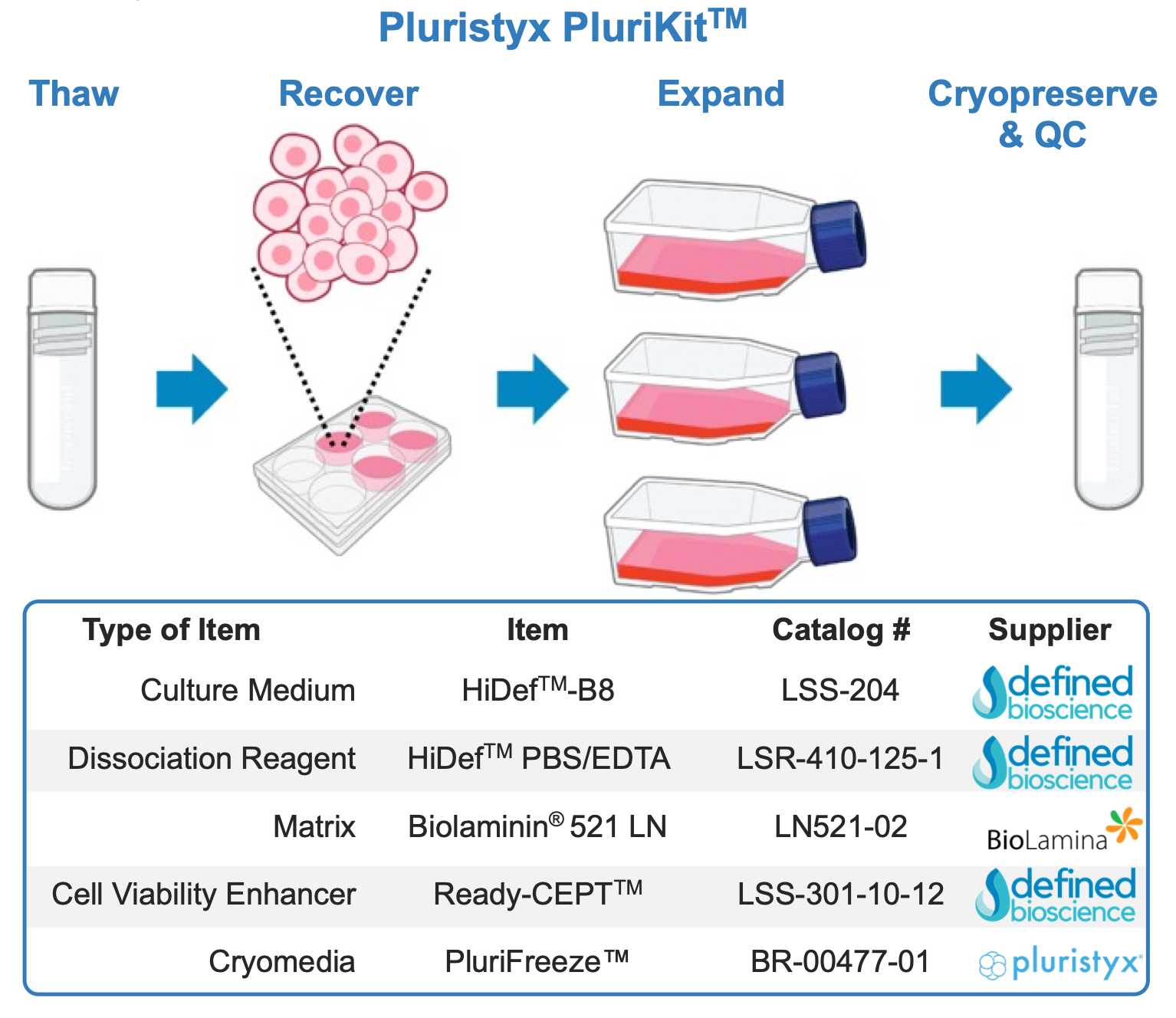
To address these challenges, Defined Bioscience and Pluristyx collaborated to develop a streamlined validated protocol, integrating a carefully selected combination of reagents to optimize hPSC culture. This work forms the foundation of PluriKit™, a validated set of reagents and protocols designed to support the consistent growth of hPSCs, demonstrated here using clinical grade iPSC lines (PluriBanks™). These include a female (PSXi011) and a male (PSXi013) PluriBank™ from Pluristyx.
Key Reagents and Their Roles
The combination of reagents used in this workflow was chosen to provide a controlled and reproducible system for hPSC culture, ensuring optimal expansion, passaging, and cryopreservation.
- HiDefTM-B8 Stem Cell Growth Medium (Defined Bioscience) – A chemically-defined, albumin/serum-free formulation designed to support the long-term expansion of iPSCs while maintaining pluripotency. HiDef-B8 contains precisely balanced growth factors to sustain consistent self- renewal and genetic stability, reducing batch-to-batch variability(1).
- Ready-CEPTTM Cocktail (Defined Bioscience) – A cell survival enhancing cocktail combining Chroman 1, Emricasan, Polyamines, and Trans-ISRIB, which collectively enhance cell viability, improve clonal efficiency, and protect against stress- induced apoptosis. Ready-CEPT Cocktail is particularly useful during single-cell passaging, cryopreservation, and differentiation workflows, minimizing cell loss and improving recovery rates(2).
- Biolaminin® 521 LN (BioLamina) – A recombinant laminin matrix that promotes efficient adhesion and pluripotency maintenance, mimicking the in vivo stem cell niche to enhance culture consistency(3).
- HiDefTM PBS-EDTA (Defined Bioscience) – A gentle, enzyme-free dissociation reagent optimized for passaging hPSCs while preserving cellular integrity. It facilitates controlled detachment, reduces mechanical and chemical stresses, and improves post-passage survival
- PluriFreeze™ (Pluristyx) – A cell freezing (cryopreservation) medium specifically formulated to ensure high post-thaw viability and recovery of hPSCs while maintaining genomic stability.
To validate this optimized culture system, we used Pluristyx’s Clinical Grade, PluriBank™CG, lines. These cells were derived and reprogrammed according to an FDA-registered, type II drug master file, using a fully synthetic, footprint-free, reprogramming system requiring no episomal DNA, circular RNA, or virus. Furthermore, as low-passage, polyclonal cells, PluriBanks allow for significant editing, adaptation, and manipulation for end user convenience. Furthermore, PluriBanks have demonstrated differentiation into multiple cell types and tissues, including islet cells, hepatocytes, CD34+ progenitors, NK cells, neurons, and cardiomyocytes, making them a valuable model for both research and therapeutic applications.
For consistent and reproducible culture of these lines, the protocol incorporating HiDef-B8 and Ready-CEPT was validated for its ability to maintain cell viability, pluripotency, and genomic integrity. This optimized system provides a standardized approach for hPSC expansion and cryopreservation, minimizing variability and improving recovery post-thaw. The following results demonstrate the performance of this workflow in maintaining high-quality, scalable hPSC culture.
Cell Culture and Expansion
Human pluripotent stem cells (hPSCs) were cultured under defined conditions to evaluate the impact of medium exchange frequency on cell expansion, morphology, and viability. Induced hPSC line PSXi013-E P6 DSB (developmental seed bank) was thawed and seeded at 20,000 cells/cm² onto Biolaminin® 521 (BioLamina)-coated T75 flasks. Cells were maintained in HiDef™-B8 Stem Cell Growth Medium (Defined Bioscience) and passaged using HiDef PBS-EDTA (Defined Bioscience), a non- enzymatic dissociation reagent. To reduce selective pressure and protect cell survival during thawing and passaging, cultures were supplemented with Ready-CEPT (Defined Bioscience), a small-molecule cocktail designed to support viability and reduce apoptosis and anoikis.
To assess the effects of different medium exchange schedules, two conditions were tested:
- Daily feeding (HiDef-B8 Daily) – Medium was completely replaced every 24 hours to maintain a consistent supply of nutrients and growth factors and removal of waste products.
- Skip-day feeding (HiDef-B8 Skip-Day) – Medium was replaced only on Day 1 and Day 4, skipping two consecutive days (Days 2 and 3). This condition was tested to determine whether a reduced medium exchange schedule could sustain hiPSC growth while minimizing labor and reagent usage.
After thaw and an initial adaptation phase (P1, Figure 1), cells were expanded through three additional passages (P2-P4) after thaw under their respective conditions. Cells were passaged every four days, with an expected doubling time of 18-24 hours.
Cryopreservation and Thawing
At P5 after thaw, cells were harvested and cryopreserved in PluriFreeze (Pluristyx), an optimized cryopreservation medium designed for hPSCs. Cells were frozen using a controlled-rate freezing protocol and stored in vapor-phase liquid nitrogen.
To evaluate post-thaw recovery, cells were thawed following a standardized protocol:
- Rapid thawing in a 37°C water bath until only a small ice pellet remained.
- Slow dilution in pre-warmed HiDef-B8 medium supplemented with Ready-CEPT, minimizing osmotic shock.
- Seeding onto rhLN521-coated plates to promote efficient attachment and recovery.
Quality Control and Characterization
To assess the reproducibility and robustness of this workflow, cells were evaluated across multiple passages and post-thaw conditions. Key quality control metrics included:
- Viability – Measured immediately post-thaw and post-passage using live/dead staining and automated cell counting.
- Pluripotency Marker Expression – Flow cytometry was used to confirm the retention of OCT4, SOX2, and SSEA4 protein expression, ensuring maintenance of the stem cell phenotype.
- Genomic Stability – Copy number variation (CNV) analysis was performed to verify the genetic integrity of iPSCs throughout expansion and post-thaw recovery.
The success criteria for this workflow included ≥70% post-thaw viability, ≥50% live cell recovery, and retention of ≥70% OCT4⁺ SOX2⁺ SSEA4⁺ cells (Figure 1). The results of this study provide insights into the feasibility of reducing media change frequency while maintaining robust hiPSC culture conditions.
Cell Morphology and Growth Under Daily vs. Skip- Day Feeding
To assess the impact of daily vs. skip-day feeding regimens, brightfield images of PSXi011-D and PSXi013-E iPSC lines were taken at Day 1 and Day 4 of passage 4 (P4) (Figure 2A). At Day 1, cells in both conditions demonstrated robust attachment and characteristic hiPSC colony morphology, with no visible differences in cell density or confluency. By Day 4, cultures in both conditions had expanded significantly, forming tightly packed colonies. No major morphological differences were observed between the daily and skip-day conditions, indicating that both feeding schedules effectively supported hiPSC growth and maintenance.
Cell Viability, Expansion, and Doubling Time Analysis
To quantitatively compare growth performance across conditions, cell viability, fold expansion, cell density at harvest, and doubling time were measured using three passages as biological replicates (Figure 2B).
- Cell Viability at Harvest: Both PSXi011-D and PSXi013-E exhibited high post-harvest viability, exceeding 90% in both daily and skip-day conditions, with no significant differences between groups.
- Fold Expansion: On average, both cell lines achieved an ≥8- fold expansion per passage, meeting expectations for robust hPSC culture. No statistically significant differences were observed between daily and skip-day conditions.
- Cell Density at Harvest: By the end of each passage, cell densities remained comparable across conditions, indicating that reduced feeding frequency did not negatively impact proliferation rates.
- Doubling Time: Both conditions maintained an average doubling time of 18-24 hours, in line with standard hPSC growth expectations.
Overall, these results confirm that the defined reagent paradigm, incorporating HiDef-B8 and Ready-CEPT, supports reproducible iPSC expansion while enabling flexible feeding schedules. The skip-day feeding approach successfully sustained hPSC growth without compromising cell viability, expansion efficiency, or genomic stability, providing a potential strategy to reduce handling time and media consumption in routine culture.
Cryopreservation and Post-Thaw Recovery
To assess the effectiveness of the combined reagent paradigm for cryopreservation and recovery, cells were frozen at P5 using PluriFreeze cryomedia and thawed under standard conditions. Key post-thaw metrics, including cell viability and live cell recovery, were measured across both daily and skip-day feeding conditions (Figure 3).
Viability at thaw remained high across conditions, with both PSXi011-D and PSXi013-E maintaining ≥80% post-thaw viability, exceeding the 70% viability threshold (blue dotted line). This indicates that the defined workflow supports high survival rates upon thawing, regardless of the media change frequency leading up to cryopreservation. Live cell recovery, calculated as viable cells counted at thaw relative to live cells loaded at freezing, remained above 60% for all conditions, exceeding the 50% live cell recovery threshold (blue dotted line). For PSXi011-D, both daily and skip-day conditions maintained recovery rates above 70%, indicating that the reduced feeding schedule did not negatively impact post-thaw recovery. For PSXi013-E, while recovery was slightly lower in the skip-day condition, it remained within an acceptable range for hPSC cryopreservation.
These results demonstrate that the HiDef-B8 and Ready-CEPT paradigm provides a robust and reproducible approach for hPSC cryopreservation, ensuring high viability and reliable post-thaw recovery across both cell lines. The slight differences in live cell recovery between conditions suggest that feeding frequency before cryopreservation may influence post-thaw outcomes for some cell lines, emphasizing the importance of optimizing culture conditions based on specific experimental needs.
Pluripotency Marker Expression and Genomic Stability
To confirm the retention of pluripotency following cryopreservation and recovery, flow cytometry analysis was performed on PSXi011-D and PSXi013-E iPSCs four days post- thaw (Figure 4A). Both cell lines maintained high protein expression levels of key pluripotency markers, including OCT4, SOX2, NANOG, and SSEA-4, across both daily and skip-day feeding conditions. The percentage of positive cells remained above the expected threshold (dotted line), indicating that the combined reagent paradigm preserves the stem cell phenotype post-thaw with no detectable loss of pluripotency markers.
To assess genomic stability, copy number variation (CNV) analysis was performed on PSXi013-E cultured under both daily and skip-day conditions (Figure 4B). The results showed normal copy number values across all chromosomes, with no detectable gains or losses. These findings confirm that the HiDef-B8 and Ready-CEPT workflow maintains genomic integrity, supporting its reliability for sustained hPSC culture and banking.
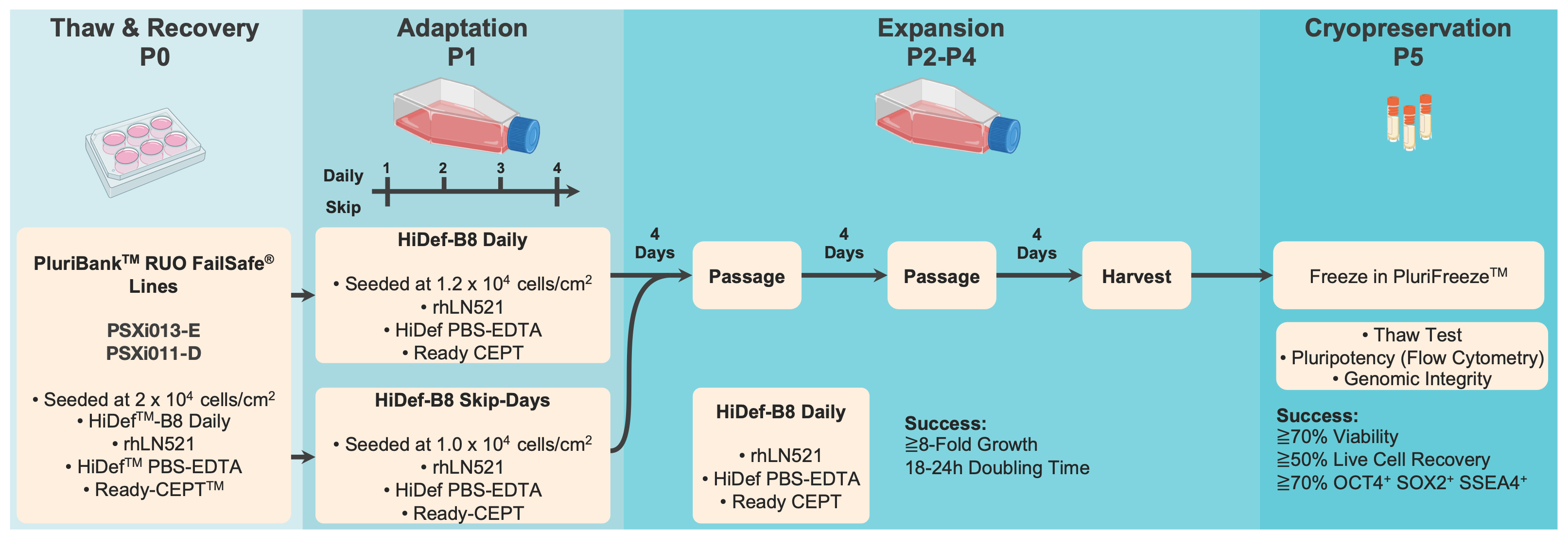
Figure 1. Schematic of the experimental workflow for hPSC expansion, cryopreservation, and recovery. All passages (P0 to P5) are counted after cells were thawed from a low passage 5 or 6 (P5 or P6) PluriBank
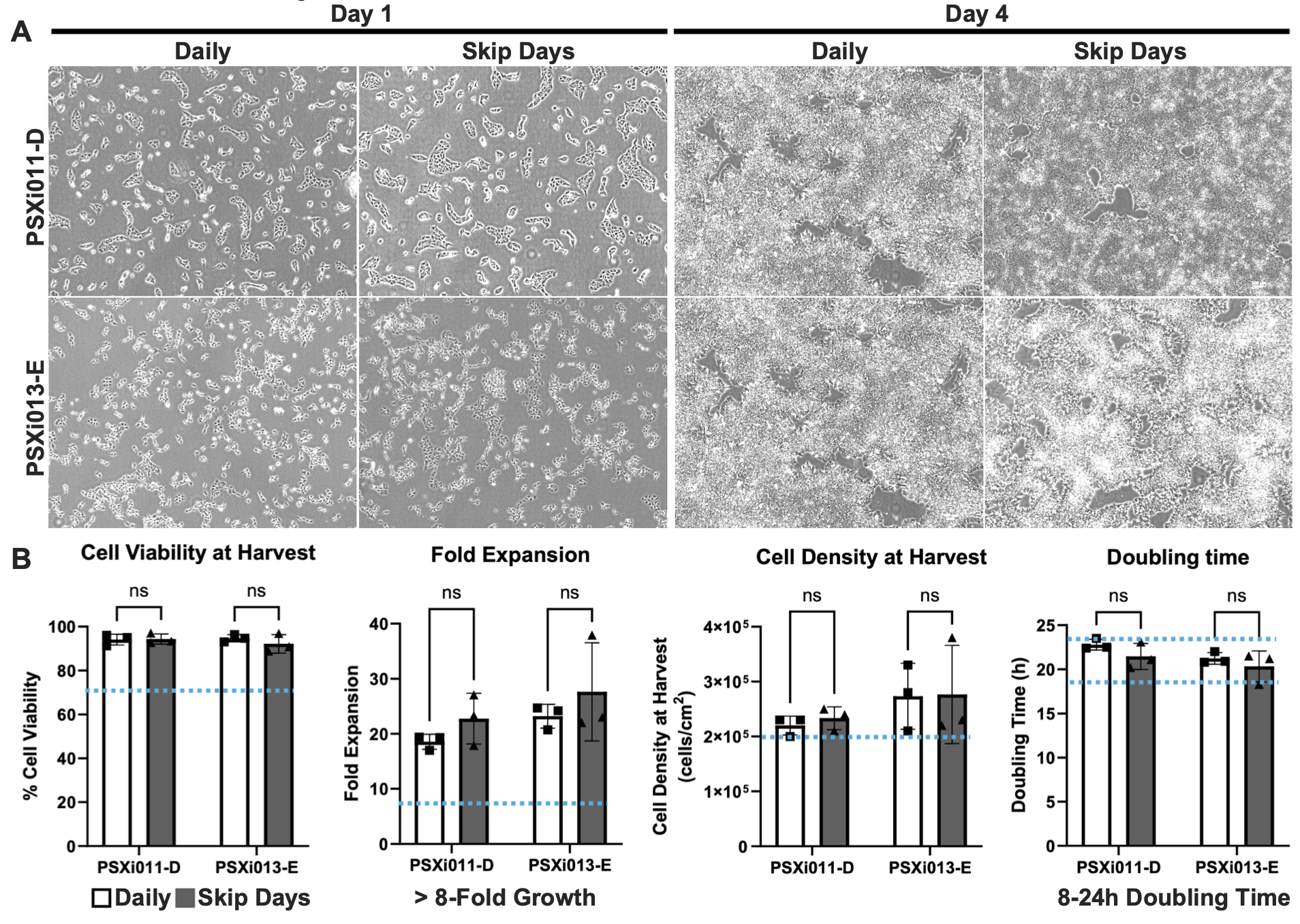
Figure 2. Brightfield images of PSXi011-D and PSXi013-E iPSCs at Day 1 and Day 4 of passage 4 (P4) under daily and skip-day feeding conditions (A). Quantitative analysis of viability, expansion, cell density, and doubling time (B) shows no significant differences, confirming both feeding regimens support robust hiPSC growth.
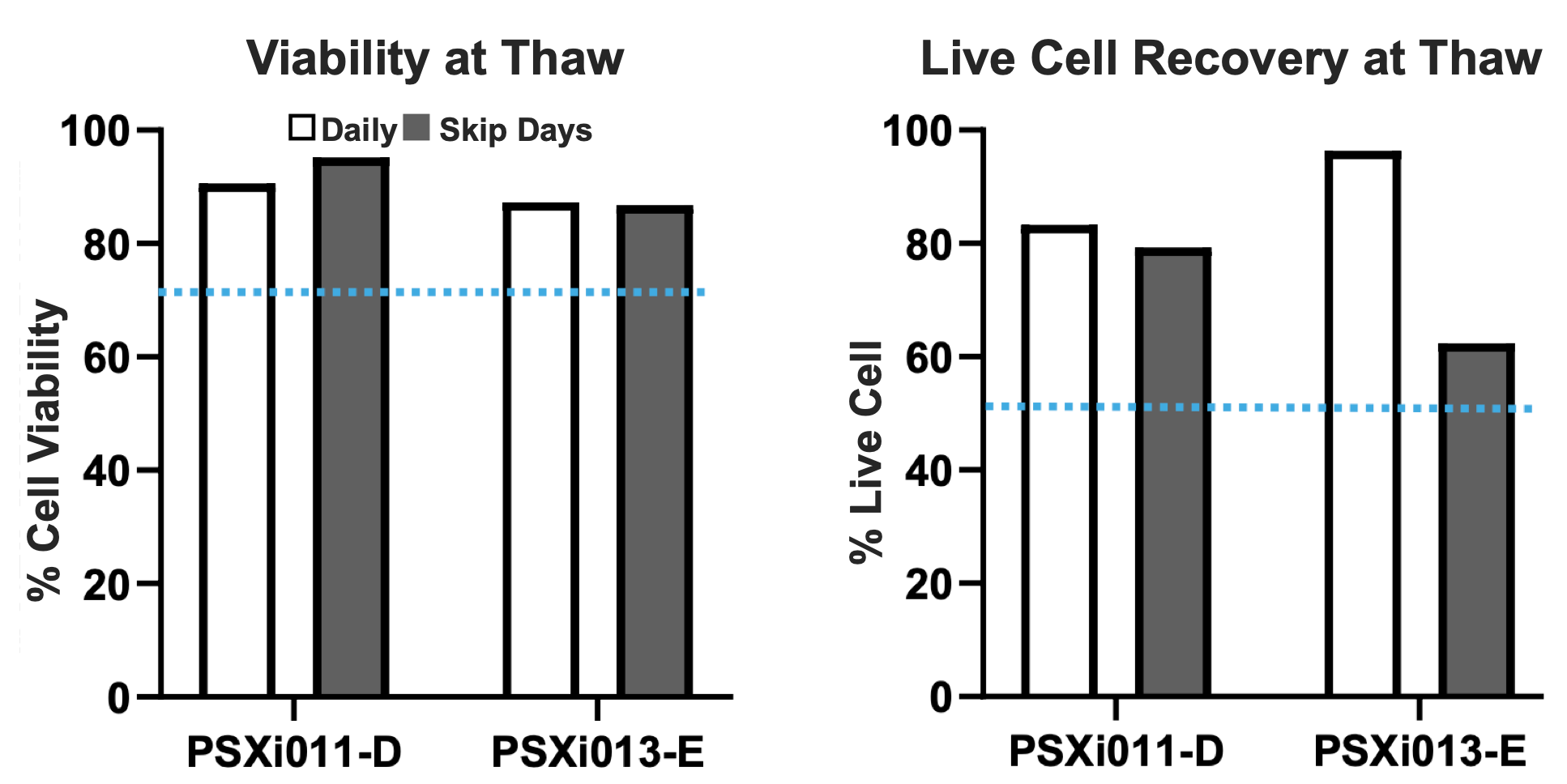
Figure 3. Post-thaw viability and live cell recovery of PSXi011-D and PSXi013-E after cryopreservation at passage 5. All conditions maintained ≥80% viability and ≥60% live cell recovery, demonstrating effective cryopreservation and recovery across feeding regimens.
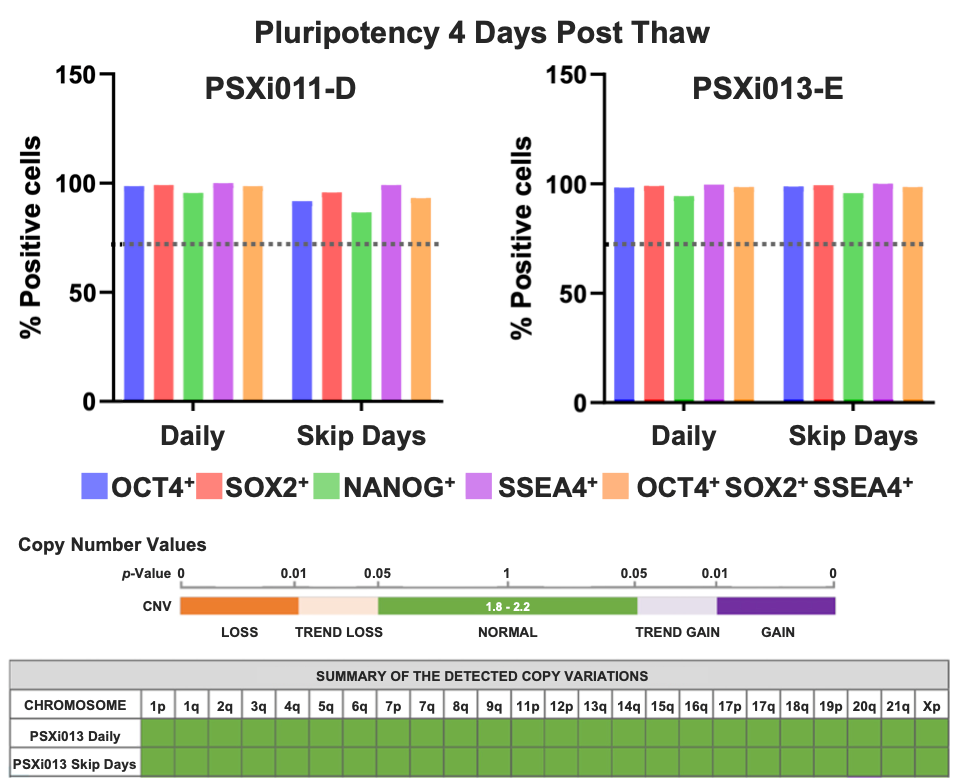
Figure 4. Assessment of pluripotency marker expression and genomic stability in FailSafe® iPSCs post-thaw. (A) Flow cytometry analysis shows retention of key pluripotency markers (OCT4, SOX2, NANOG, and SSEA-4) in PSXi011-D and PSXi013-E iPSCs four days after thaw, with no significant differences between daily and skip-day conditions. (B) Copy number variation (CNV) analysis of PSXi013-E confirms normal genomic integrity across all tested chromosomes, demonstrating that the defined reagent system preserves both pluripotency and genetic stability after cryopreservation.
This study demonstrates that the HiDef-B8 and Ready-CEPT workflow, in combination with PluriFreeze cryopreservation, supports robust hPSC expansion, cryopreservation, and post- thaw recovery. Both daily and skip-day feeding regimens maintained high viability, consistent expansion, and stable pluripotency marker expression, confirming the reproducibility of the system. Post-thaw assessments further verified that the protocol preserves cell survival, pluripotency, and genomic integrity, making it a reliable and scalable solution for hiPSC culture and banking. Additionally, this study highlights the compatibility of this workflow with Pluristyx’s Clinical Grade PluriBank™ lines, demonstrating a standardized and efficient approach for maintaining iPSC for use as a raw material to make product cells, including manufacturing of living-medicines. These findings showcase a flexible and optimized strategy for high- quality hPSC culture while improving handling efficiency.
If you're interested in learning more, we’d love to hear from you.
- Kuo, H. H., Gao, X., DeKeyser, J. M., et al. (2023). Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Reports.
- Tristan, C. A., Hong, H., Jethmalani, Y., et al. (2022). Efficient and safe single-cell cloning of human pluripotent stem cells using the CEPT cocktail. Nature Protocols. https://doi.org/10.1038/s41596-022-00753-z
- Rodin, S., Antonsson, L., Niaudet, C., et al. (2014). Clonal culturing of human embryonic stem cells on laminin-521/E- cadherin matrix in defined and xeno-free environment. Nature Communications, 5, 3195. https://doi.org/10.1038/ncomms4195
HiDef™ and Ready-CEPT™ are trademarks of Defined Bioscience.
PluriKit™, PluriBank™, and PluriFreeze™ are trademarks of Pluristyx. FailSafe® is a registered trademark of Pluristyx.
Biolaminin® is a registered trademark of BioLamina.

HiDef® B8 Stem Cell Growth Medium
HiDef® B8 Stem Cell Growth Medium
Choose your option

DMEM/F12 Basal Medium
DMEM/F12 Basal Medium
Choose your option
Ready-CEPT® Cell Viability Enhancer
Ready-CEPT® Cell Viability Enhancer
Choose your option