High-density pluripotent stem cell expansion in a single-use bioreactor using HiDef S8

Defined Bioscience Inc, San Diego, CA
- Gerardo Aguirre Castillo
- Jerome V Karpiak1

Distek Inc, North Brunswick, NJ
- Justin Cesmat
Human pluripotent stem cell (hPSC) technology, including induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), has been transformative for biomedical science. hPSCs can be maintained and expanded in vitro due to their self- renewing capacity and virtually unlimited proliferative potential(1). As a result, hPSC-derived cells are increasingly used in disease modeling, high throughput drug screening, and the development of emerging cell therapies(2), including iPSC-derived natural killer (NK) cells, cardiomyocytes, neural lineages, and other clinically relevant cell types. These applications often require large quantities of undifferentiated hPSCs grown under consistent, defined conditions suitable for translational or manufacturing environments.
Historically, hPSC expansion has relied on adherence- dependent culture systems, which involve coating plasticware with extracellular matrix proteins or ligand peptides and scaling out to increase yield. More recently, three-dimensional suspension culture in stirred bioreactors has emerged as a preferred approach due to its capacity to support higher cell densities, improved nutrient and gas exchange, and easier scalability by increasing culture volume(3). Still, challenges such as aggregate size control, shear effects, and batch-to-batch variability remain significant barriers to efficient suspension culture(4).
In this work, we describe the development of a scalable suspension culture workflow established through a collaboration between Defined Bioscience and Distek® that supports large- scale, 3D expansion of hPSC aggregates in a single-use bioreactor. Central to this workflow is HiDefTM S8TM, a new chemically defined, animal-free suspension medium developed specifically for the efficient expansion and maintenance of hPSCs. Designed as the ‘next-gen’ formulation building on the success of HiDef ® -B8, which was originally optimized for feeder- free 2D adherent culture, HiDef ® S8™ facilitates robust hPSC manufacture in bioreactors without the need for microcarriers or serum supplementation.
Using the 3D workflow described herein, we achieved a 60-fold expansion of hPSC within a single five-day run. For comparison, 2D adherent expansion over a single four-day passage yielded 23-fold vs. 29-fold for 3D suspension over the same duration. This demonstrates the potential of HiDef-S8 to facilitate scale-up, reduce cost, and streamline stem cell bioprocessing for both research and therapeutic applications.

Cell Culture and Expansion
A two-liter, BIOne single-use bioreactor (SUB, Distek) was used as the 3D suspension expansion platform for this study (Graphical Abstract). This BIOne SUB featured a single right- handed pitch blade impeller and a microsparger (20-40 µm pore size) serving also as a cell strainer for spent medium removal during exchange. The bioreactor working volume was defined as 1200 mL for the expansion run. This working volume was defined as it resulted in an operational system aspect ratio of 1:1 (liquid height:diameter). The bioreactor system was operated using the BIOne 1250 Bioprocess Control Station (Distek).
Parent adherent hPSC cultures were expanded in 2D under standard conditions (37°C / 5% CO2 / atmospheric O2), using a commercially available animal-free defined formulation (HiDef- B8, Defined Bioscience) specifically developed for the maintenance of pluripotent stem cells in 2D (Fig. 1A, left panel). Cells were initially expanded in 2D cultures, then enzymatically dissociated for bioreactor inoculation at 1.0 x 105 cells/mL, sufficient to establish 3D aggregates (Fig. 1B, right panel).
The hPSCs were cultured as free-floating aggregates using HiDef-S8 (Defined Bioscience) supplemented with optional 1% polyvinyl alcohol (PVA, Sigma-Aldrich) and initially inoculated in the presence of Ready-CEPT™ cocktail (Defined Bioscience). Operating parameters for the bioreactor were as follows:
temperature at 37°C, pH at 7.20 ± 0.1, and dissolved oxygen (DO) at 40%. Initial agitation speed was set at 170 rpm (5.37 W/m3) with downward axial flow, then increased to 250 rpm (17.08 W/m3) within two hours of inoculation. The agitation strategy was determined through iterative hPSC inoculation testing within the BIOne SUB, aiming to minimize power input and shear forces on the cells while still providing enough mixing to prevent large visible cell mass from accumulating in the system.
Due to concerns about shear induced single-cell damage from sparged gas bubbles bursting, an overlay-only gassing strategy was selected for initial aggregate formation. The baseline gassing supply rate was set at 130 sccm (0.1 vvm) introducing air, N2, and CO2. Supplemental air and N2 gassing were programmed within the DO cascade and CO2 gassing was programmed within the pH cascade to maintain 40% DO and other process parameters at set points for the hPSC expansion process.
The hPSC process was continued for 5 days. Medium exchange was initiated 48 hours post-inoculation, with continuous perfusion starting at 0.5 vessel volumes per day (VVD, 600 mL) using HiDef-S8 plus PVA, without Ready-CEPT. Each subsequent 24 hours, the exchange rate was increased by an additional 0.5 VVD (Fig. 2). Spent media was removed through the microsparger and fresh media was added through an addition port on the headplate of the bioreactor at the same rate as media removal. The run was terminated after five days.
Samples were taken twice daily from the bioreactor via a Luer- lock sample port. Agitation remained enabled during sampling. To account for the holdup volume in the sample line, 4 mL was discarded before 2 mL of the culture was collected and assessed for morphology, size, viability and metabolic activity. Upon completion of bioreactor expansion, hPSCs were harvested and then replated in 2D with HiDef-B8 for further expansion and pluripotency analysis by flow cytometry, or cryopreserved.
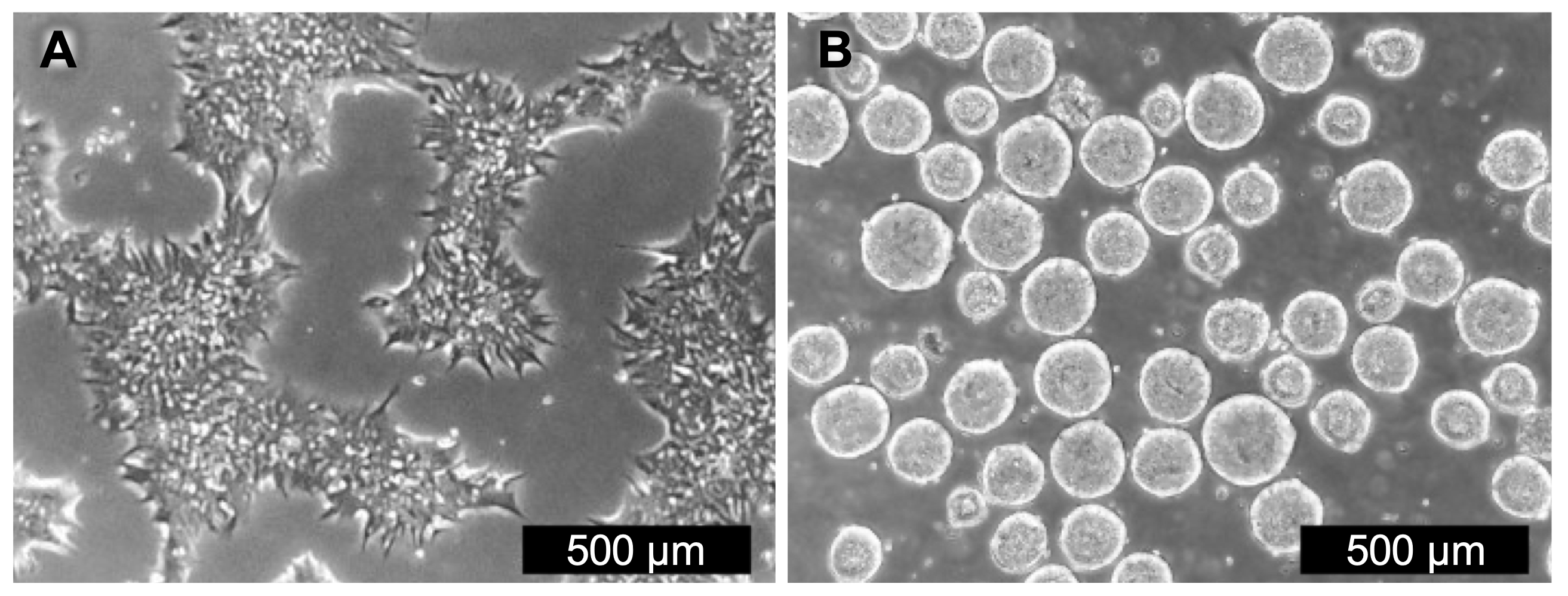
Figure 1. hPSC workflows in parallel A. Substrate-based adherent cultures were grown in HiDef-B8 medium (Day 2). B. Suspension cultures were inoculated with 1.2 x 108 cells, or 1.0 x 105 cells/mL (Day 5).
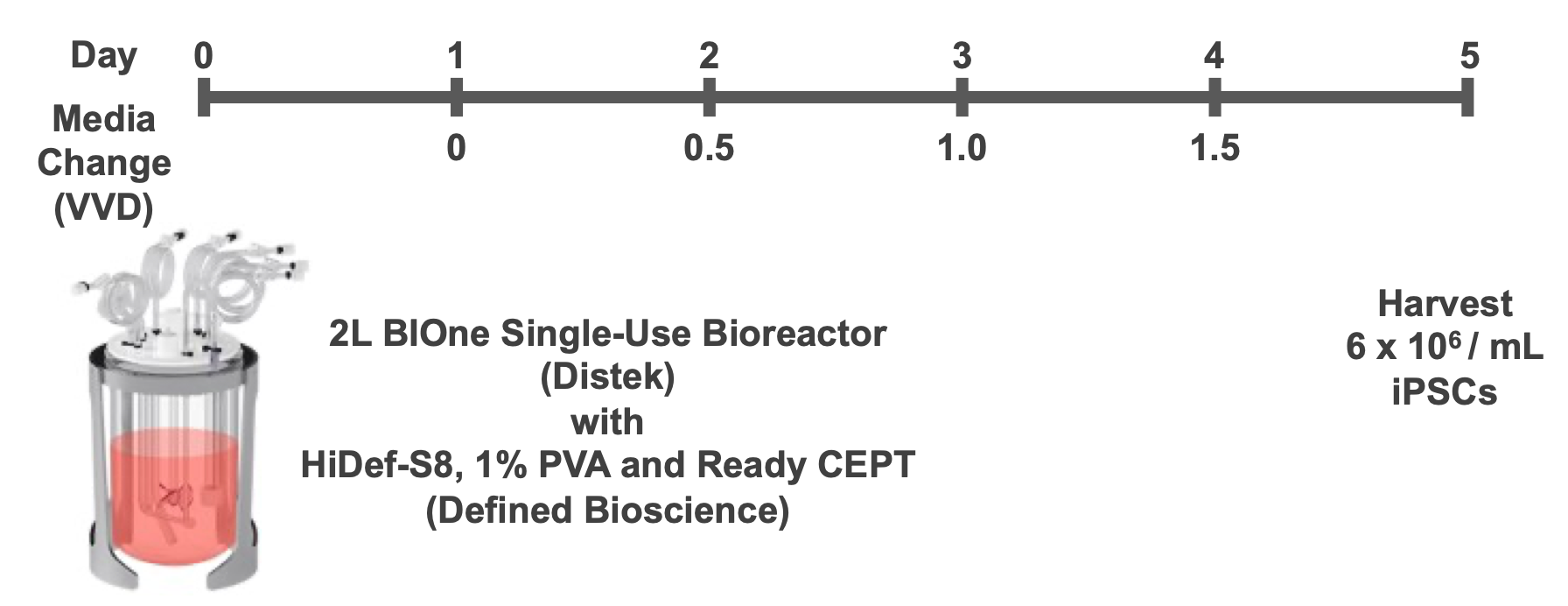
Figure 2. 3D Suspension Culture Overview. Five days of suspension culture in the 2L BIOne Single-Use Bioreactor using HiDef-S8 with 1% PVA. Inoculated with Ready-CEPT cocktail, fresh HiDef-S8 exchange started at Day 2 and resulted in 60-fold expansion to over 7 billion hPSCs.
3D Suspension Culture
Cell viability and aggregate size were balanced by adjusting the agitation speed, resulting in consistent aggregate diameters below 200 µm and high viability. The hPSCs grown in 3D suspension formed aggregates that linearly increased in size from approximately 50 µm in diameter on Day 1 of culture to approximately 150 µm diameter by Day 5 (Fig. 3A). Over the five days of suspension culture, the number of cells increased exponentially in the culture system, resulting in a 60-fold expansion (7.23 x 109 cells) by the fifth day of culture (Fig. 3B). In addition to this accelerated rate of growth, cells within the aggregates maintained a high viability in excess of 94% throughout five days of suspension culture. (Fig. 3C). Population doubling time was 19.7 through Day 4, without the typical “ra p- up” exhibited in adherent workflows, co pared to 21.3 hours in parallel 2D cultures over the same duration. This demonstrated a substantial difference in cells produced per unit of medium spent, resulting in cost savings over D2-D5 (Fig. 3D).
The improved growth observed during this five-day expansion is further highlighted in Fig. 4. Notably, 3D suspension cultures expanded faster than parallel cultures in 2D (Fig. 4). Moreover, the efficiency of cells produced (number of cells produced per volume of spent medium) was also higher in 3D culture relative to 2D and reached ~1.5 million cells generated per mL of medium consumed in 3D suspension culture. This resulted in a projected supplement cost of US$0.30 per million cells for this line using the Distek-Defined Bioscience platform.
2D Adaptation & Pluripotency Marker Expression
Importantly, transition of bioreactor 3D aggregates in HiDef-S8 back to 2D using HiDef-B8 did not require any special transition effort. When 3D-expanded hPSC aggregates were directly plated onto a 2D substrate and cultured using HiDef-B8, they acquired identical morphology to monolayer expanded cells within 24 hours and maintained this over ten passages (Fig. 5).
Quantification of pluripotency markers using flow cytometry demonstrated that hPSCs re-plated and cultured in 2D from suspension aggregates co-express surface markers Tra-1-60 and SSEA-4 at unchanged levels (Fig. 6). Similarly, aggregates derived from the bioreactor were frozen, thawed, and cultured for several additional passages, leading to the co-expression of Tra- 1-60 and SSEA-4, again at levels unchanged (data not shown). Qualitatively, morphology of the re-plated cells was the same as they presented prior to suspension cultures with an archetypical hPSC growth pattern (Fig. 1A & Fig. 5).
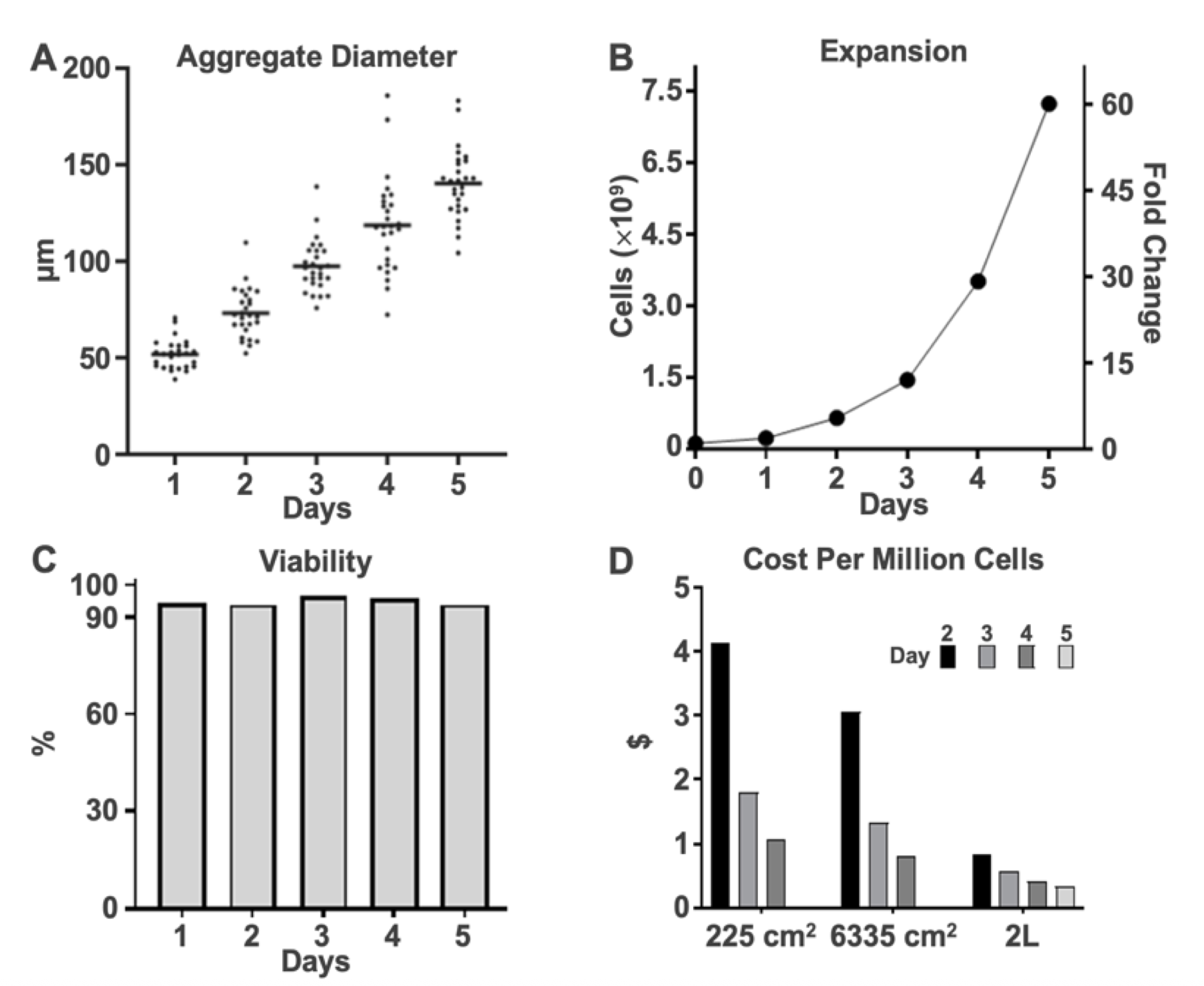
Figure 3. Three-Dimensional Suspension Culture Is More Efficient. A. Stirring was optimized for aggregate size to support rapid growth while preserving viability and pluripotency. B. Cell expansion increased exponentially over a five-day run reaching >7 billion cells in 1,200 mL. C. Cell viability remained >94% over five days. D. Efficiency and cost effectiveness of the HiDef-S8 supplement resulted in a price per million cells generated of less than US$0.30, lower than using HiDef-B8 supplement for 2D expansion on T-225 and Corning® CellSTACK® surfaces.
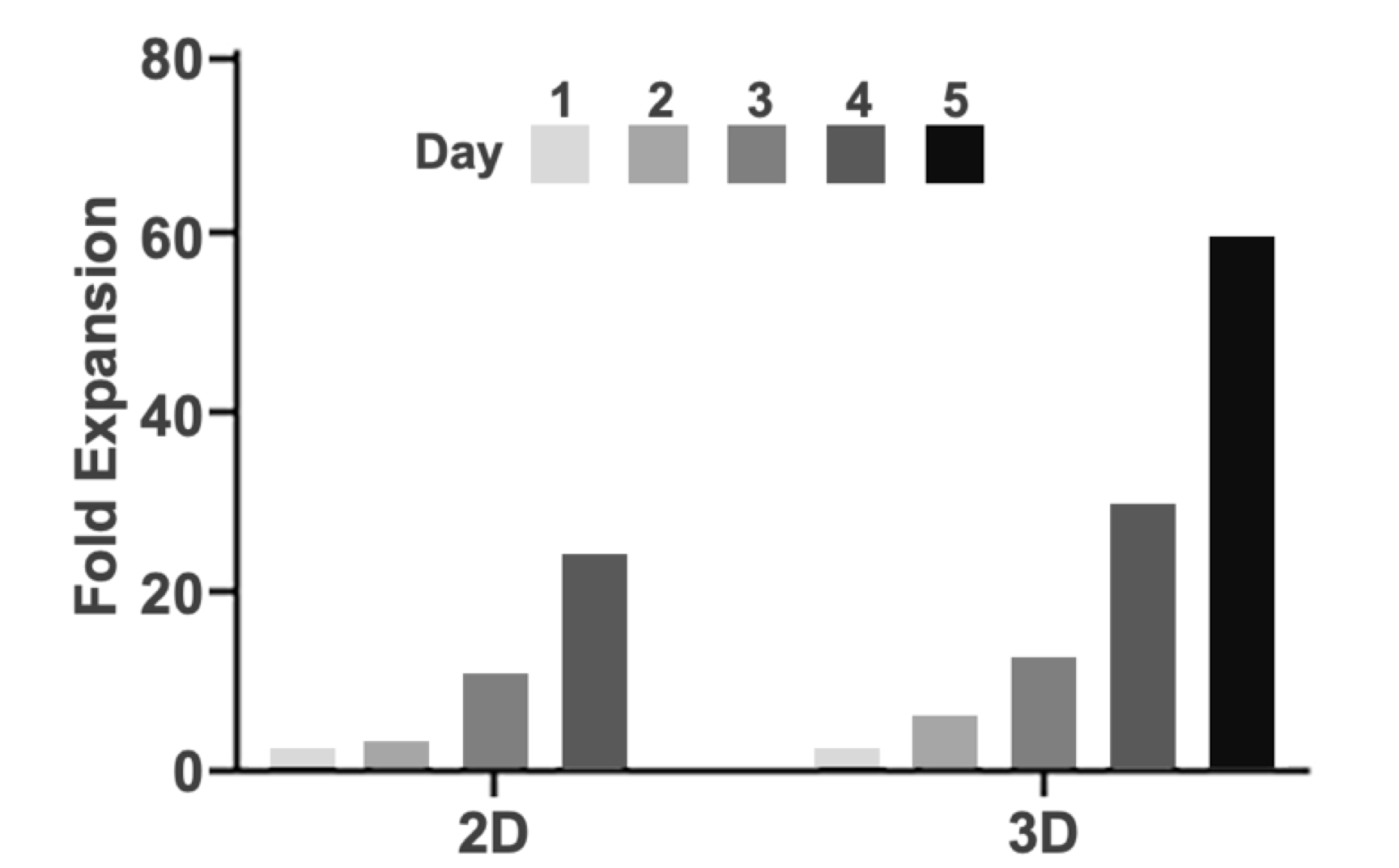
Figure 4. Higher fold expansion in suspension cultures. Fold expansion comparison between 2D and 3D cultures.
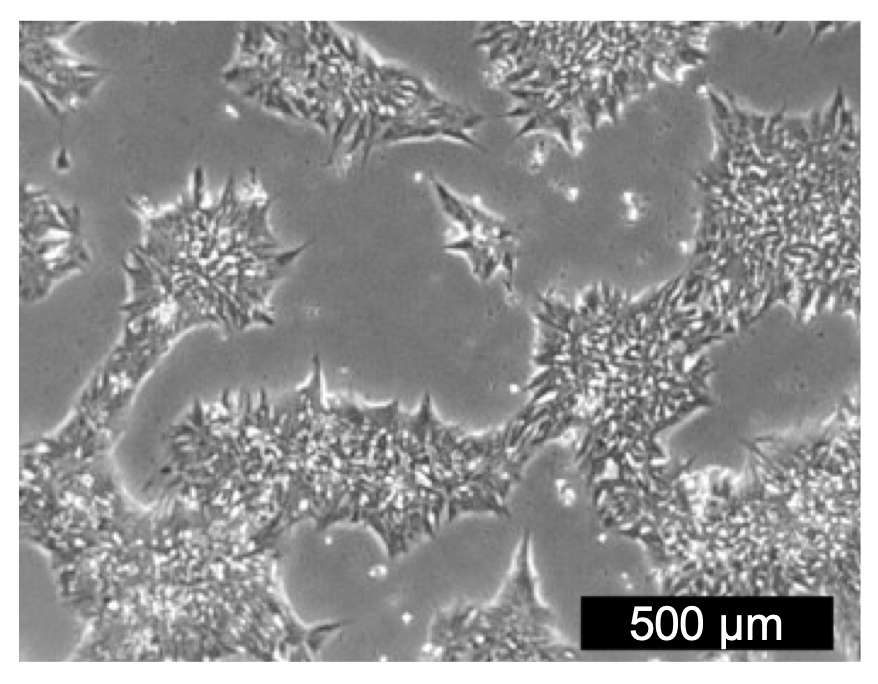
Figure 5. Suspension-expanded cells retain 2D hPSC morphology. Following five days of suspension culture in HiDef-S8, cell aggregates were re-plated in 2D without dissociation to observe morphology. These cells were cultured in HiDef- B8 for ten passages and exhibited identical morphology to monolayer- only expanded cells, within 24 hours.
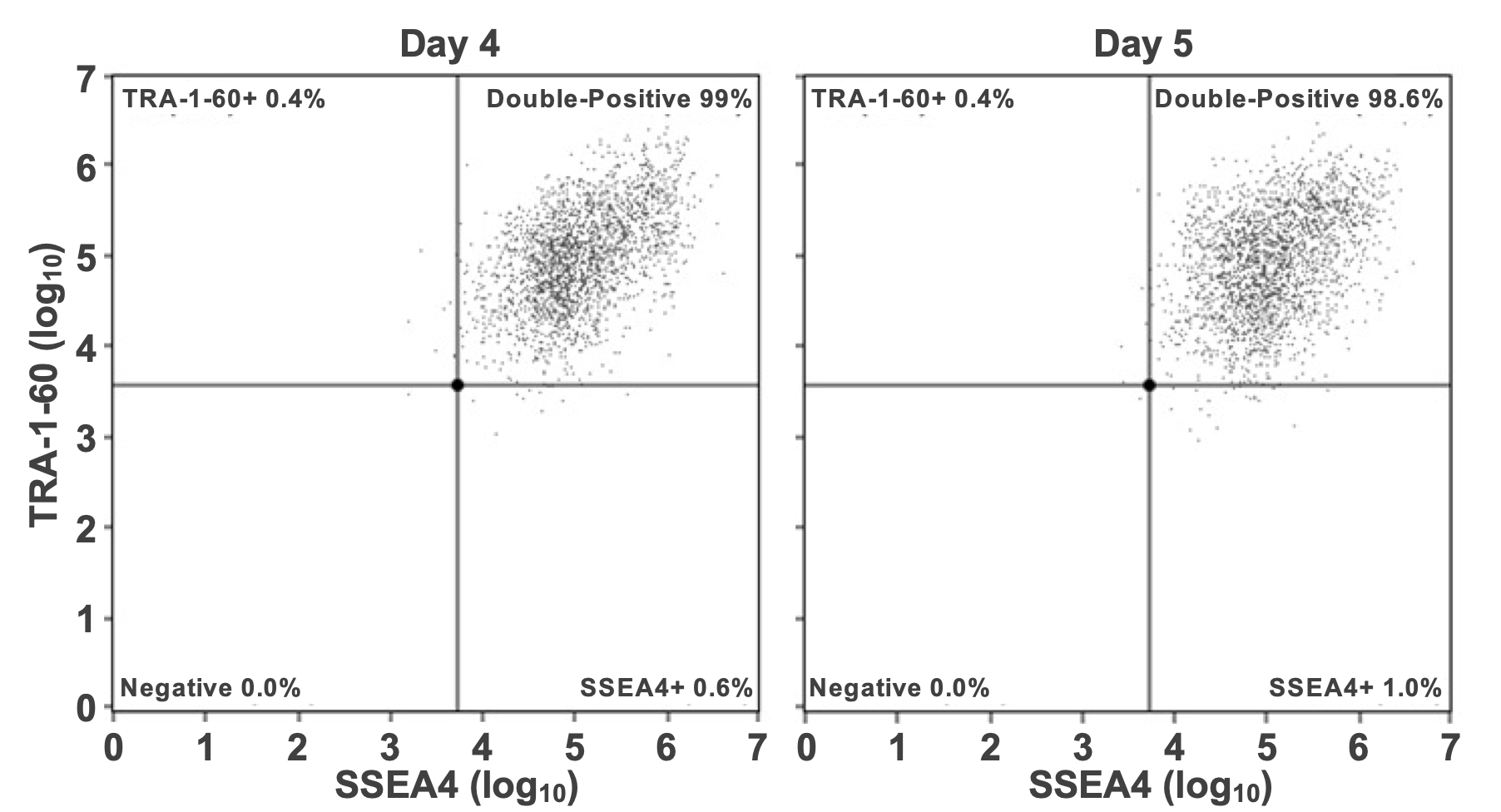
Figure 6. Suspension culture retains high pluripotency. Flow cytometric analysis for Days 4 and 5 suspension cultures hPSC aggregates. These were re-plated in 2D adherent culture using HiDef-B8 to quantify pluripotency marker expression after ten passages.
This study demonstrates that HiDef-S8, with Ready-CEPT, supports rapid, high yield, carrier-free expansion of hPSCs in a stirred tank suspension bioreactor. In just five days, seven billion cells were generated in a two-liter BIOne single use bioreactor system operating at half-capacity, while maintaining high viability and expression of key pluripotency markers. The expanded hPSCs readily re-attached in 2D adherent culture, maintained normal morphology, and retained pluripotency after multiple passages and freeze thaw cycles.
HiDef-S8 was specifically developed to meet the challenges of large-scale suspension culture and builds on the proven performance of HiDef-B8. Its defined, animal-free composition provides a reliable and scalable platform for seamlessly transitioning hPSC workflows from conventional monolayer cultures to high efficiency bioreactor systems. By eliminating the need for microcarriers and minimizing process complexity, HiDef- S8 reduces cost per cell, improves yield per unit of media, and supports the quality requirements of both research and translational applications.
As demand grows for hPSC-based therapies and engineered cell products such as natural killer (NK) cells, T cells, cardiomyocytes, and neural lineages, scalable solutions as described herein become essential. The co bination of Distek’s robust bioreactor and controller technologies with Defined Bioscience’s HiDef-S8 suspension medium offers a practical and cost-effective strategy for modern stem cell bioprocessing.
If you're interested in learning more, we’d love to hear from you.
- Cerneckis J, Cai H, Shi Y. Induced pluripotent stem cells (iPSCs): molecular mechanisms of induction and applications. Signal Transduct Target Ther. 2024;9(1):112. doi:10.1038/s41392-024-01809-0
- Colter J, Murari K, Biernaskie J, Kallos MS. Induced pluripotency in the context of stem cell expansion bioprocess development, optimization, and manufacturing: a roadmap to the clinic. npj Regen Med. 2021;6(1):72. doi:10.1038/s41536-021-00183-7
- Cuesta-Gomez N, Verhoeff K, Dadheech N, et al. Suspension culture improves iPSC expansion and pluripotency phenotype. Stem Cell Res Ther. 2023;14(1):154. doi:10.1186/s13287-023-03382-9
- Yehya H, Raudins S, Padmanabhan R, Jensen J, Bukys MA. Addressing bioreactor hiPSC aggregate stability, maintenance and scaleup challenges using a design of experiment approach. Stem Cell Res Ther. 2024;15(1):191. doi:10.1186/s13287-024-03802-4
HiDef ® is a registered trademark of Defined Bioscience,Inc.
Ready-CEPTTM and S8TM are trademarks of Defined Bioscience, Inc.
Distek® is a registered trademark of Distek, Inc.
Corning® and CellSTACK® are registered trademarks of Corning, Inc.

HiDef® B8 Stem Cell Growth Medium
HiDef® B8 Stem Cell Growth Medium
Choose your option